The 2010 Olympic gold-medal downhill skier Lindsey Vonn was recently (March 8, 2010) interviewed in the magazine Time. Lindsey Vonn was the first American woman to win gold in an Olympic downhill, and she did so while being injured. One of the questions posed to her was:
“How did you find the strength to ski with an injury?”
Her response was, in part:
“It’s been a real challenge for me to be able to ski well despite this injury. I’ve been doing as much therapy as I can.”
“And I do laser therapy and massage.”
•••••
The healing of injured musculoskeletal tissue is primarily done by the formation of a protein patch. The primary repair protein is the connective tissue collagen. The primary cell that creates the collagen repair is the connective tissue fibrocyte cell.
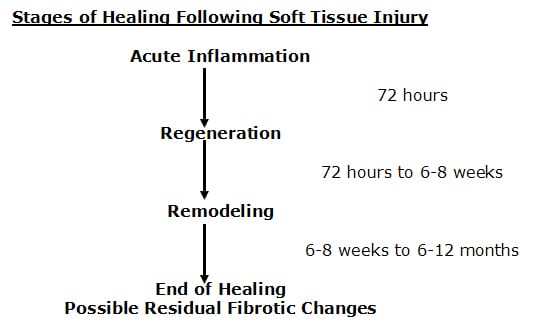
Healing of injured musculoskeletal tissues takes place in three specific phases. A review of these phases is as follows (Kellett 1986):
The first phase is called the acute inflammatory phase. This phase will last approximately 72 hours. After the initial injury, an electrical current is generated at the wound, called the “current of injury.” This “current of injury” attracts fibroblasts to the wound (Oschman, 2000). During this phase there is also initial bleeding and continual associated inflammation of the injured tissues. Because of the increasing inflammatory cascade during this period of time, it is not uncommon for the patient to feel worse for each of the first three days following injury. Because there is disruption of local vascular supplies, there is insufficient availability of substrate (glucose, oxygen, etc.) to produce large enough quantities of ATP energy to initiate collagen protein synthesis to repair the wound.
After about 72 hours following injury, the damaged blood vessels have repaired. The resulting increased availability of glucose and oxygen elevates local ATP levels and collagen repair begins by the fibroblasts that accumulated during the acute inflammatory phase. This second phase of healing is called the phase of regeneration. During the regeneration phase the disruption in the injured muscles and ligaments is bridged. This phase will last approximately 6-8 weeks (Jackson, 1977). At the end of 6-8 weeks, the gap in the torn tissues is more than 90% bridged.
There is a third and final phase of healing. This phase is called the phase of remodeling. The phase of remodeling starts near the end of the phase of regeneration. During the phase of remodeling the collagen protein glues that have been laid down for repair are remodeled in the direction of stress and strain. This means that the fibers in the tissue will become stronger, and will change their orientation from an irregular pattern to a more regular pattern, a pattern more like the original undamaged tissues.
It is established that remodeling takes place as a direct byproduct of motion. Traditional chiropractic management of injured musculoskeletal tissues involves the application of controlled motion into those tissues and joints. Consequently, chiropractic management has its greatest influence on the third phase of healing, the phase of remodeling. This is reflected in the studies that show that chiropractic spinal adjusting (specific directional manipulation) achieves its greatest clinical improvement in patients that are suffering from chronic problems and who have failed to achieve an acceptable clinical outcome from other approaches to management (Kirkaldy-Willis 1985, Meade 1990, Woodward 1996, Khan 1999, Giles 2003). The important question for this discussion is this:
Is there a safe, effective approach to enhance the timing and quality of musculoskeletal healing that targets the second phase of the response, the phase of regeneration?
Key to this discussion, and it is important to restate, is that ATP energy is required to synthesize repair proteins. In 1997, Douglas Wallace wrote an article for Scientific American titled “Mitochondrial DNA In Aging and Disease.” In this article, he notes that an intracellular organelle, the mitochondria, is the power plant of cells because it produces ATP energy. “Mitochondria provide about 90% of the energy that cells, and thus tissues, organs and the body as a whole, need to function.” Every cell in the body contains hundreds of mitochondria that produce the energy that the body requires.
Synthesizing repair proteins occurs primarily in the phase of regeneration. Attachment of a single amino acid onto the string of amino acids that will become a protein requires the availability and expenditure of 4 ATP molecules (Champe 1994). Also, additional ATP molecules are required for both initiation and termination of the amino acid chain synthesis. The development of repair proteins is limited by the availability of ATP energy.
A summary of the genesis of ATP energy is as follows:
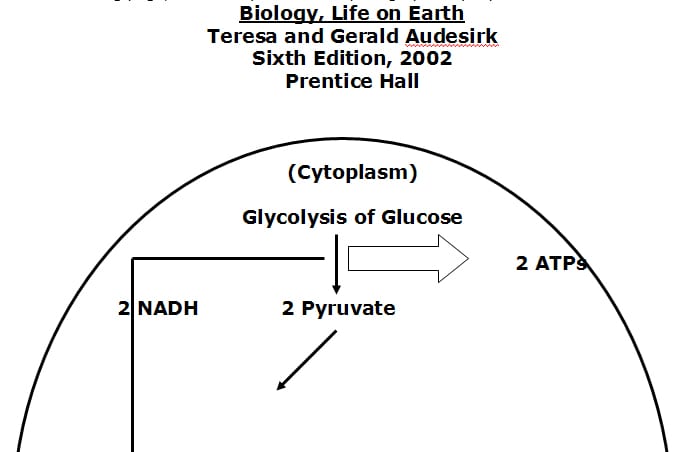
• The two variable but absolute substances required for ATP genesis are glucose and oxygen.
• Without adequate oxygen the cytoplasm of the cell can produce 2 ATP molecules per glucose molecule. This is called anaerobic glycolosis. Aerobic life cannot be sustained through the anaerobically derived quantity of ATP energy.
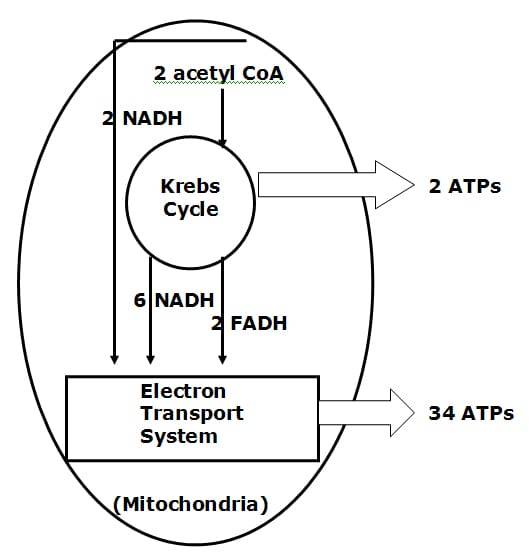
• With oxygen, additional ATP energy is formed in an intracellular organelle called the mitochondria.
• With adequate oxygen, the mitochondrial Kreb’s Cycle can produce 2 more ATP molecules per original glucose molecule. Simultaneously, the Kreb’s Cycle is generating the all important electron transport proteins, which are shunted to the inner membrane of the mitochondria, know as the electron transport system (chain).
• The electron transport chain of the mitochondria produces the majority of ATP molecules, an astonishing 34 per glucose molecule. Function and efficiency of the electron transport chain is also oxygen dependent.
On the following page, a summary of these steps is graphically represented:
Things that compromise the synthesis of ATP energy will impair healing. As an example, smoking cigarettes. The carbon monoxide in cigarette smoke has a greater affinity for hemoglobin than does oxygen. Smoking reduces the delivery of oxygen to the cells, impairing both the Kreb’s Cycle and electron transport chain genesis of ATP energy. Consequently, smokers heal more slowly and less completely. They suffer from accelerated tissue degradation and degeneration as a consequence of reduced ability to replicate proteins.
In contrast, things that enhance tissue oxygenation will enhance the genesis of ATP energy, in turn enhancing the genesis of repair proteins. Some examples of this include:
• Yogi breathing exercises (Weil 1996).
Improved breathing results in improved tissue oxygenation, improving the genesis of mitochondrial (Kreb’s Cycle and electron transport chain) ATP, enhancing the timing and the quality of the genesis of repair proteins, accelerating healing.
• Being aerobically fit.
The number of mitochondria one has per cell is not a constant, it is a variable. The more aerobically fit one is the more mitochondria one will have per cell. The more mitochondria one has, the more efficient one is at utilizing glucose and oxygen in generating mitochondrial (Kreb’s Cycle and electron transport chain) ATP energy. More ATP enhances the timing and the quality of the genesis of repair proteins, accelerating healing.
There is another variable controlling the mitochondrial genesis of ATP energy that is becoming increasingly noticed and documented in the National Library of Medicine literature database: it is an enzyme called:
cytochrome c oxidase
The cytochrome c oxidase enzyme is a terminal enzyme of the mitochondrial electron transport chain. In fact, it is the rate-limiting enzyme in the production of ATP energy by the electron transport system. Upregulating or increasing the activity of the cytochrome c oxidase will proportionally increase the genesis of ATP energy. How can the treating doctor upregulate or increase the activity of the cytochrome c oxidase enzyme? A portion of the answer is the understanding that “chrome” pertains to color. The remainder of the answer is Low Level Laser Therapy.
Laser light is different than background environmental light. In the electromagnetic spectrum, visible background environmental light exists between wavelengths of about 400 – 800 nanometers (nm). Different wavelengths will produce different colors. As examples, 410 nm is a blue color, 535 nm is a green color, and 635 nm is a red color. Background environmental light is a combination of all wavelengths, and therefore of all colors. Laser light is monochromatic. This means laser light is of a single wavelength and therefore a single color. Laser eliminates all wavelengths but one.
Background environmental light is also scattered, meaning the light is spread in all directions. In contrast, laser light is coherent. This means that the light is not scattered. All of the light waves are brought to a single point.
As a consequence of laser light’s monochromatism and coherency, laser light can elicit interesting biological and physiological responses.
In the understanding of low level laser physiology, it is important to restate that the primary producer of ATP energy is the electron transport system of the mitochondria. The inner membrane of the mitochondria contains 4 protein complexes called the respiratory chain. Electrons from food pass through these protein complexes with the help of Coenzyme Q10, interacting with oxygen and hydrogen to produce water and ATP energy.
As the respiratory chain participates in ATP energy production, toxic by-products known as oxygen free radicals are generated. These free radicals can attack all components of cells, including respiratory chain proteins and mitochondrial DNA. Anything that impedes the flow of electrons through the respiratory chain can increase their transfer to oxygen molecules and promote the generation of free radicals. Importantly, anything that improves the flow of electrons through the respiratory chain will increases the production of ATP while reducing the generation of free radicals.
Tiina Karu, PhD, is the world’s leading authority on low level laser therapy. Dr. Karu is Head of the Laboratory of Laser Biology and Medicine, Institute on Laser and Informatic Technologies of the Russian Academy of Science. Dr. Karu holds two PhD’s; one in Science and Biophysics, and another in Photochemistry. Pertaining to laser biophysics and laser physiology, Dr. Karu has authored 3 books, 16 book chapters, and 146 journal articles.
Dr. Karu wrote “Low-Power Laser Therapy” in Biomedical Photonics Handbook, in 2003. She notes that low-level laser therapy probably works because the laser light is absorbed by the mitochondria photoreceptors, which enhances cellular metabolism. This means the mitochondria produce more ATP as a result of exposure to laser light. She notes that the primary reaction of laser light is in the mitochondria, which results in increased ATP energy. Dr. Karu states:
“The mechanism of low-power laser therapy at the cellular level is based on the increase of oxidative metabolism of mitochondria, which is caused by electronic excitation of components of the respiratory chain.”
“It is known that even small changes in ATP level can significantly alter cellular metabolism.”
“Our results also provide evidence that various wavelengths (670, 632.8, and 820 nm) can be used for increasing respiratory activity.”
“The photobiological action mechanism via activation of the respiratory chain is a universal mechanism” [for laser light effects].
In 2007, Dr. Karu wrote a book titled:
Ten Lectures on Basic Science of Laser Phototherapy
This book is perhaps the most detailed accounting of the biological and physiological basis for low-level laser therapy published to date. Dr. Karu reviews how laser radiation can modulate cell metabolism through the mediation of a universal photoreceptor, the cytochrome c oxidase enzyme. Once again, the cytochrome c oxidase enzyme is the enzyme that catalyzes the final step in the mitochondrial respiratory chain, thereby becoming the rate-limiting step in the genesis of ATP energy. In this book, Dr. Karu makes the following points:
“Cytochrome c oxidase plays a central role in the bioenergetics of the cell.”
“It is well known that it is only in the ideal case where the supply of electrons from cytochrome c oxidase is unlimited that electrons are always present to reduce oxygen,” thus maximizing production of ATP and minimizing production of oxygen free radicals.
“One of the most important biological mechanisms [for low level laser therapy is] based on activation/upgrading of the terminal enzyme of the mitochondrial respiratory chain, cytochrome c oxidase, is shown to be a universal mechanism controlling many aspects of the metabolism in different types of [laser] irradiated cells.”
“The activation of cells via the mitochondria is suggested to be a universal photobiological mechanism.”
“Laser phototherapy is used by physiotherapists (to treat a wide variety of acute and chronic musculoskeletal aches and pains), by dentists (to treat inflamed oral tissues and to heal diverse ulceration), by dermatologists (to treat edema, indolent ulcers, burns, and dermatitis), by rheumatologists (to relieve pain and treat chronic inflammation and autoimmune diseases), and by other specialists, as well as by general practitioners. Laser phototherapy is also widely used in veterinary medicine (especially in racehorse-training centers) and in sports medicine and rehabilitation clinics (to reduce acute swelling and hematoma, relieve pain, improve mobility, and treat acute soft-tissue injuries.”
•••••
Another reference text pertaining to low-level laser therapy was published in 2002 by Jan Turner and Lars Hode, titled:
Laser Therapy Clinical Practice and Scientific Background
This book contains 1,281 references. These authors note:
1) “Today, we can safely say that therapeutic lasers have an important biological effect, and a very positive one at that.”
2) “We believe that lasers have a tremendous and as yet untapped potential in the field of healthcare.”
3) “Therapeutic lasers have no undesirable side effects in the hands of a reasonably qualified therapist.”
4) Lasers are “sterile, painless, and often less expensive than methods already in use,” and does not have side effects as does pharmacotherapy.
5) “Laser therapy of wounds is ideal, since it promotes healing and reduces pain at the same time.”
6) Laser light increases the cell’s ATP energy.
In their text, Turner and Hode note that the first company to receive a 510(k) market clearance from the Federal Drug Administration (FDA) was a US company, Erchonia Laser. This occurred in 2002. There are now (as of March 28, 2010) 7 FDA market clearances for low-level laser therapy, Erchonia obtaining 5 of the 7. Erchonia is located in McKinney, TX, and they can be contacted at phone number (214) 544-2227 or web address www.erchonia.com.
•••••
A recent (March 28, 2010) search of the National Library of Medicine database using the PubMed search engine and the words “low-level laser therapy” brought up 2,204 titles. Two representative articles pertaining to low level laser therapy and tissue healing are reviewed below:
Effects of Helium-Neon Laser on Levels of Stress Protein and Arthritic Histopathology in Experimental Osteoarthritis
American Journal of Physical Medicine & Rehabilitation
October 2004, 83(10):758-765
Lin, Yueh-Shuang MS; Huang, Mao-Hsiung MD, PhD; Chai, Chee-Yin MD, PhD; Yang, Rei-Cheng MD, PhD
In this study, researchers injured the knees of 42 rats giving them arthritis. Twenty-one of the rats were given 632 nm low-level laser (red color), applied over the arthritic knee for 15 minutes, three times per week, for 8 weeks; the other 21 rats were not similarly exposed. The results showed a marked repair of arthritic cartilage in the lased rats, but not in the non-lased group. The authors concluded that the 632 nm low-power laser enhances protein production in arthritic joints and repairs the arthritic cartilage.
These authors also note: Laser is “thought to cause electronic excitation of the photoacceptor molecules, which are thought to be various cytochrome enzymes that are terminal electron carriers in the respiratory chain.” This is thought to accelerate electron transfer. “Electron transport in the mitochondrial membrane is one of the main fueling mechanisms underpinning metabolism and proliferation of cells, including generation of adenosine triphosphate (ATP).” “Low-level laser mediated increase in efficiency of the electron carriers in the respiratory chain would increase generation of adenosine triphosphate, which could manifest itself as increased DNA and protein synthesis and result in cell proliferation, as shown in present study.”
In summary, and consistent with the explanations above, these researchers credited the outcomes of this animal study to laser driven excitation of the cytochrome enzyme terminal electron carriers of the mitochondrial respiratory chain, increasing ATP synthesis and enhancing repair protein production.
•••••
Therapeutic low powered lasers are commercially available in many different wavelengths and power outputs. It appears clear that there is no one wavelength that is ideal for all appropriately treated clinical syndromes. Few studies compare the outcome of different wavelengths and exposure fluences on measured outcomes. However, the second reviewed article did just that:
Comparative Study Using 685-nm and 830-nm Lasers in the Tissue Repair of Tenotomized Tendons in the Mouse
Photomedicine and Laser Surgery
December 2006, Volume 24, Number 6, pp. 754–758
Carrinho PM, Renno ACM, Koeke P, Salate ACB, Parizotto NA, Vidal BC
In this study, the authors compared the tissue repair of injured mouse tendons when treated with either a 685 nm laser or an 830 nm laser, each at fluences of both 3 J/cm2 and 10 J/cm2. This study used 48 mice that were divided into six experimental groups:
Group A, tenomized animals, treated with 685 nm laser, at the dosage of
3 J/cm2.
Group B, tenomized animals, treated with 685-nm laser, at the dosage of
10 J/cm2.
Group C, tenomized animals, treated with 830-nm laser, at dosage of 3 J/cm2.
Group D, tenomized animals, treated with 830-nm laser, at dosage of 10 J/cm2.
Group E, injured control (placebo treatment).
Group F, non-injured standard control.
Laser irradiation started 24 hours after the tenotomy of the Achilles tendon. A total of 12 laser sessions were performed on consecutive days. The rats were killed on day 13, and the injured tendons were surgically removed and analyzed with polarized light microscopy to analyze the organization and molecular order of the collagen fibers. All laser treated groups showed improved healing when compared to injured control group. The best organization and aggregation of the collagen bundles was shown by the animals of group A (685 nm, 3 J/cm2), followed by the animals of group C (830 nm, 3 J/cm2), and B (685 nm, 10 J/cm2), and finally, the animals of group D (830 nm, 10 J/cm2). The authors concluded:
“All wavelengths and fluences used in this study were efficient at accelerating the healing process of Achilles tendon post-tenotomy, particularly after the 685-nm laser irradiation, at 3 J/cm2. It suggests the existence of wavelength tissue specificity and dose dependency.”
Interestingly, in this study, the shorter wavelength was associated with the better healing outcome. Counterintuitively, lesser exposure to laser irradiation resulted in an improved healing outcome than higher doses of exposure. These authors note:
“The better tissue response was observed after the irradiation with the 685-nm laser, at the dosage of 3 J/ cm2.”
“The animals irradiated with the 830-nm laser, at the dosage of 10 J/ cm2 presented the weaker response to laser irradiation.”
“The best tissue response was obtained after the 685-nm laser irradiation, at the dosage of 3 J/cm2.”
Specifically, the 685-nm laser irradiation at 3 J/cm2 showed (the shortest of the compared wavelengths and the lowest amount of irriadence):
16% improved tendon healing over the 830-nm laser at 3 J/cm2.
33% improved tendon healing over the 685-nm laser at 10 J/cm2.
54% improved tendon healing over the 830-nm laser at 10 J/cm2.
Compared to the control tendons, the improved tissue response was as follows:
101% improved tissue response with the 830-nm laser at 10 J/cm2.
114% improved tissue response with 685-nm laser at 10 J/cm2
167% improved tissue response with the 830-nm laser at 3 J/cm2.
208% improved tissue response with 685-nm laser irradiation at 3 J/cm2
These authors concluded:
“Our results suggest that laser irradiation produced an increase of cell proliferation through changes in mitochondrial physiology, subsequently affecting RNA synthesis, which, in turn, alters the expression of various cell regulatory proteins.”
•••••
In summary, the evidence suggests that low level laser therapy targets the mitochondrial enzyme that is the rate-limiting factor in the production of ATP energy, the cytochrome c oxidase enzyme. The increased production of ATP energy allows the DNA to enhance the replication of repair proteins, accelerating healing and improving symptoms. Since lasers can achieve this without side effects or risks, low-level laser therapy is here to stay. As a consequence, many chiropractors now incorporate low level laser therapy as a component of their patient clinical management, especially of injured patients.
References:
Audesirk T, Audesirk G. Biology, Life on Earth Sixth Edition, Prentice Hall 2002.
Carrinho PM, Renno ACM, Koeke P, Salate ACB, Parizotto NA, Vidal BC; Comparative Study Using 685-nm and 830-nm Lasers in the Tissue Repair of Tenotomized Tendons in the Mouse; Photomedicine and Laser Surgery; December 2006, Volume 24, Number 6, pp. 754–758.
Champe PC, Havey RA. Lippincott’s Illustrated Reviews: Biochemistry, Second Edition, Lippincoll Eilliams & Wilkins, 1994.
Giles LGF, Muller R. Chronic Spinal Pain: A Randomized Clinical Trial Comparing Medication, Acupuncture, and Spinal Manipulation. Spine. July 15, 2003; 28(14):1490-1502.
Jackson, R. The Cervical Syndrome, Fourth Edition, Thomas, 1977.
Karu T. “Low-Power Laser Therapy” Chapter 48 in Biomedical Photonics Handbook, Tuan Vo-Dinh, CRS Press, 2003.
Karu T. Ten Lectures on Basic Science of Laser Phototherapy. Prima Books
2007.
Kellett, John, “Acute soft tissue injuries-a review of the literature,” Medicine and Science of Sports and Exercise, American College of Sports Medicine, Vol. 18 No.5, (1986), pp 489-500.
Khan S, Cook J, Gargan MF, Bannister GC. A symptomatic classification of whiplash injury and the implications for treatment. The Journal of Orthopaedic Medicine 21(l) 1999, 22-25.
Kirkaldy-Willis, W.H., M.D., & Cassidy, J.D., “Spinal Manipulation in the Treatment of Low-Back Pain,” Canadian Family Physician, (1985), 31:535-40.
Lin, Yueh-Shuang MS; Huang, Mao-Hsiung MD, PhD; Chai, Chee-Yin MD, PhD; Yang, Rei-Cheng MD, PhD, Effects of Helium-Neon Laser on Levels of Stress Protein and Arthritic Histopathology in Experimental Osteoarthritis. American Journal of Physical Medicine & Rehabilitation. 83(10):758-765, October 2004.
Meade TW, Dyer S, Browne W, Townsend J, Frank AO. Low back pain of mechanical origin: Randomized comparison of chiropractic and hospital outpatient treatment. British Medical Journal. Volume 300, JUNE 2, 1990, pp. 1431-7.
Oschman, James L, Energy Medicine: The Scientific Basis, Churchill Livingstone, 2000.
Turner J, Hode L, Laser Therapy Clinical Practice and Scientific Background, Prima Books, 2002.
Wallace D, Scientific American, Mitochondrial DNA In Aging and Disease, Scientific American, August 1997.
Weil A. Spontaneous Healing. Ballantine Books, 1996.
Woodward NM, Cook JHC, Gargan MF, Bannister GC. Chiropractic treatment of chronic ‘whiplash’ injuries. Injury. Volume 27, Issue 9, November 1996, Pages 643-645.
Thousands of Doctors of Chiropractic across the United States and Canada have taken "The ChiroTrust Pledge":
“To the best of my ability, I agree to
provide my patients convenient, affordable,
and mainstream Chiropractic care.
I will not use unnecessary long-term
treatment plans and/or therapies.”
To locate a Doctor of Chiropractic who has taken The ChiroTrust Pledge, google "The ChiroTrust Pledge" and the name of a town in quotes.
(example: "ChiroTrust Pledge" "Olympia, WA")
Content Courtesy of Chiro-Trust.org. All Rights Reserved.